What Does It Mean to Be a Group or Family on the Periodic Table
Core Concepts:
In this tutorial, you volition learn how to read the periodic table. Nosotros will have a close look at the groups of the periodic tabular array. In addition, you will learn almost the unlike properties of the periodic table groups, periods, and families. If you enjoy this article, be certain to check out our others!
Related Articles:
- Periodic Trends Made Piece of cake
- Elements
- How to Write Electron Shell Configurations
- Atomic Radius Tendency
- Ionization Energy Trend
Vocabulary
- Elements: A pure substance composed of a unmarried cantlet.
- Groups: The vertical column of the periodic tabular array that signifies the number of valence electrons in an element.
- Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element.
- Families: Elements that have the same number of valence electrons and therefore like properties.
The Periodic Table and the Periodic Trends

The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). Elements in the same group have the same number of valence electrons. Meanwhile, elements in the aforementioned period have the aforementioned number of occupied electron shells. In 1869 Russian chemist Dmitri Mendeleev noticed in that location existed an innate blueprint of organisation for the chemic elements. From this deduction, he formed the periodic tabular array. It is important to annotation how the location of elements on this table tells united states about their properties. A quick way to empathise an element'south chemical and concrete properties is to know the periodic trends. These trends tell you where the highest and lowest types of properties are concentrated on the periodic table. For a more than in-depth explanation of periodic trends, click here.
Grouping vs Period
Groups are the columns of the periodic table, and periods are the rows. There are 18 groups, and there are 7 periods plus the lanthanides and actinides.
Periods on the Periodic Table
So what is a period on the periodic table? Periods are the horizontal rows of the periodic table. There are seven periods total and each element in a menstruum has the same number of atomic orbitals. The height menstruum, which contains hydrogen and helium, has only 2 orbitals. As you lot get downwardly the rows, the number of orbitals increases. Below is a table to help visuals the periodic number and the corresponding orbitals.
| Menstruum Number | Number of Orbitals | Number of Elements |
| 1 | 1 | ii |
| 2 | two | 8 |
| 3 | 3 | 8 |
| 4 | 4 | eighteen |
| 5 | five | 18 |
| vi | six | 32 |
| vii | seven | 32 |
Groups of the Periodic Table
As previously mentioned, the vertical columns on the periodic table are called "groups". There is eighteen groups on the periodic table in total, and each periodic table group contains elements with the same number of valence electrons.
The number of valence electrons present dictates the properties of an chemical element. The reason for this is that the valence electrons, which are the electrons in the outermost shell, are the ones taking function in chemical reactions. These electrons are either donating, accepting, or sharing. Moreover, the more filled the valence trounce is, the more stable the element.
How many groups are in the periodic table?
There are 18 groups in the periodic table, one per each column of the periodic table. The first column on the left is group 1, and the last column on the correct is group eighteen.
Groups and Valence Electrons
The beginning group is the least stable as information technology only has i valence electron. Meanwhile, group eighteen is the about stable every bit these elements have a full valence beat out (eight valence electrons). Below is a table relating the group numbers to the number of valence electrons.
| Group Number | Number of Valence Electrons |
| i | 1 |
| 2 | 2 |
| three-12 | 2 |
| thirteen | 3 |
| 14 | 4 |
| fifteen | five |
| xvi | vi |
| 17 | seven |
| xviii | 8 |
Families of the Periodic Tabular array
On the periodic table, in that location are families which are groups of elements with similar properties. These families are alkali metals, alkaline earth metals, transition metals, mail-transition metals, metalloids, halogens, noble metals, and noble gases. Many of these families belong to a single group on the periodic tabular array. However, not all of the families overlap with periodic table groups. For instance, the transition metals comprise all elements from grouping iii to group twelve. Below is a periodic table where displaying the location of each family.
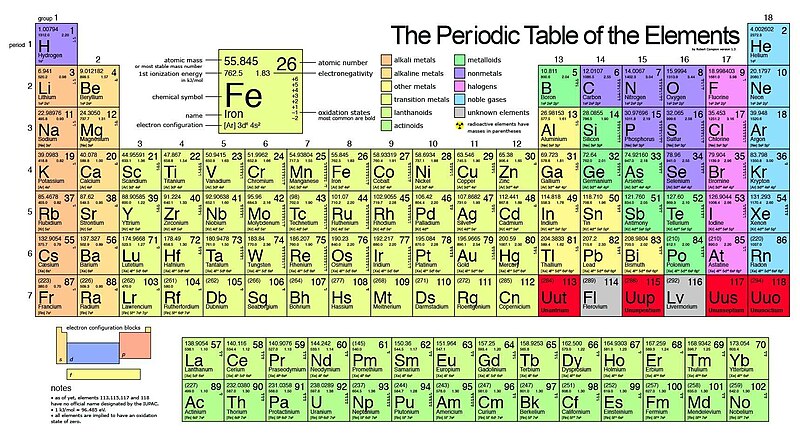
The Alkali Metals (Group i)
The alkali metals consist of all of the elements in group ane with the exception of hydrogen. These elements are extremely reactive and for this reason, are normally found in compounds. In add-on, they are water-sensitive (they react violently with water), so they must be stored in oil. The most reactive alkali metal is francium and information technology decreases as you lot go upwards the group. This means lithium is the least reactive. Physically, the alkali metal family is silvery, white, and light. They likewise have low melting and depression boiling points.
The Element of group i Globe Metals (Group ii)
The alkaline world metals are the 2d most reactive family on the periodic table (post-obit behind the alkali metals). Moreover, they are strong reducing agents which means they donate electrons in chemical reactions. They are also good thermal and electric conductors. Physically, they accept depression density, low melting signal, and a low boiling betoken.
Rare Earth Metals: Lanthanides
Lanthanides are a family of rare earth metals that contain one valence electron in the 5d shell. They are highly reactive and a strong reducing amanuensis in reactions. Furthermore, they are a silvery-bright metal and are relatively soft. They also take both loftier melting points and high boiling points. The rare earths include elements like neodymium and erbium.
Rare Earth Metals: Actinides
Actinides are another family of rare earth metals. Like the lanthanides, these elements are highly reactive. They also have loftier electropositivity and are radioactive. Additionally, these elements contain paramagnetic, pyromorphic, and allotropic properties. Physically, they are very similar to lanthanides. They are silvery metals that are soft, malleable, and ductile.
The Transition Metals (Groups three-xi)
The transition metals typically course two or more oxidation states. They have low ionization energies and high conductivity. In addition, they have high melting points, high boiling points, and high electrical conductivity. Physically they are both metallic and malleable.
Mail service Transition element
The post transition metals are located in between the transition metals and the metalloids. At standard temperature, they are in a solid country of matter. They tend to have a loftier density besides equally high conductivity. Physically they are malleable and ductile.
The Metalloids
The metalloids display properties of both metals and non-metals. For example, metals are practiced conductors and not-metals are poor conductors. This means metalloids are semiconductors (only conducts electricity at high temperatures.). Also, they are more than brittle than metals only less breakable than non-metals. Physically they can be either shiny or dull and are typically ductile and malleable.
The Halogens (Grouping 17)
The name element of group vii means "salt formers" in greek. This is evident in nature as halogens interact with metals to class various salts. On another note, the halogens are a unique group of elements. They are the only periodic family unit that contains elements in the 3 states of matter at standard temperature. At that place are 6 halogens and they are located in grouping 17. These elements include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are highly reactive, highly electronegative, and highly toxic non-metals.
Noble Metals
The noble metals consist of ruthenium (Ru), osmium (Os), rhodium (Rh), iridium (Ir), Pd, platinum (Pt), gilded (Au), silver (Ag). Like the noble gases, they are inert due to having a consummate valence beat out. In addition, noble metals have catalytic tendencies. Also, they are very resistant to corrosion, tarnishing, and oxidation. Finally, like many of the other metals, they are soft and ductile.
Noble Gases (Group 18)
The noble gases, also called aerogens, are inert gases. Some examples include argon, krypton, and neon. They can exist found in group eighteen on the periodic table. Likewise, this ways they have a complete valence shell. For this reason, they are stable and relatively unreactive. Furthermore, the noble gases have low humid points and depression melting points. Physically they are colorless and have no odour.
Summary Tabular array for Family Properties
| Family Blazon | Properties |
| Alkali Metals | – highly reactive – water-sensitive – Soft – low density – depression melting point – depression boiling point |
| Alkali metal Earth Metals | – Strong reducing agents – Silvery, shiny metal – Good conductors – Depression density – Low melting point – Low boiling betoken |
| Transition Metals | – two or more oxidation states – Usually forms paramagnetic compounds – Low ionization energies – Loftier melting indicate – High humid betoken – High conductivity – Metallic – Malleable |
| Mail Transition Metals | – Solid at standard temperature – Malleable – Ductile – High electrical conductivity – High density |
| Metalloids | – Semi-conductors (conducts merely at high temperatures) – More breakable than metals only less breakable than non-metals – Properties are a mix between metals and non-metals – Shiny or tedious – Ductile and malleable |
| Lanthanides | – ane valence electron in 5d shell – Highly reactive – Potent reducing agent – Silvery bright metal – Relatively soft – Loftier melting points – Loftier boiling points |
| Actinides | – Highly reactive – High electropositivity – Paramagnetic – Pyromorphic – Allotropic – Radioactive – Argent metals – Ductile – Malleable – Soft |
| Halogens | – Highly reactive – High electronegativity – Non-metal – Toxic |
| Noble Metals | – Relatively unreactive – Complete valence shell (viii valence electrons) – Inert – Catalytic – Resistant to corrosion, tarnishing, and oxidation – Soft and Ductile |
| Noble Gases | – Relatively unreactive – Complete valence trounce (8 valence electrons) – Low electronegativity – Colorless and odorless – gases under standard conditions – Non-metallic – Depression boiling point – Low melting bespeak – Density increases as you go down |
Further Reading
The Structure of an Cantlet
Periodic Trends Made Easy!
Source: https://chemistrytalk.org/how-to-read-the-periodic-table/
0 Response to "What Does It Mean to Be a Group or Family on the Periodic Table"
ارسال یک نظر